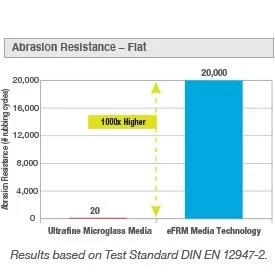
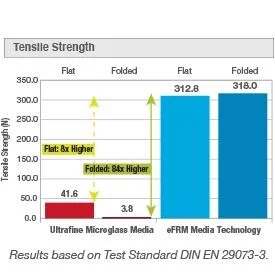
The superior mechanical strength is demonstrated by a high tensile strength, burst pressure, and abrasion resistance. eFRM Media Technology retains its integrity with a high aversion to any potential damage, such as mishaps in handling or installation. This mitigates the risk of filter media failure and additional incurred validation and repair expenses. As a result, there is a decreased risk of contaminants entering cleanroom environments. Protection of sterile products and cleanroom personnel is optimized. Improvement in quality risk management systems of critical applications ensures a consistent supply of quality products and a reduction of failure rates.
Reduce Operational Risk
The pharmaceutical industry estimates that 77% of production downtime can be attributed to failures of equipment and environmental problems. This downtime can be caused by HEPA filters failing. Traditional microglass HEPA filters typically fail due to some form of contact combined with the poor mechanical strength of the filter. The actions required when these failures occur include repairing/replacing the HEPA filter, certifying the repair or new installation, investigating potentially contaminated product, and generation of a risk assessment report. Effectively managing the risk and costs associated with successful operation requires utilizing HEPA filters with dramatically higher tensile strength that is highly resistant to chemical degradation, thereby eliminating premature leaking and failure.
Increase Uptime
While FDA Testing Guidance requires critical room leak-testing certification twice a year, non-critical rooms require testing only once a year. With the extremely high tensile strength and durability of the eFRM pleated filter media, 84 times stronger than microglass, ISO 7 and 8 areas could be tested annually. Increasing time between certifications results in less PAO exposure to the gel seal (gel degradation), lower labor costs, and increased production time.
MEGAcel II eFRM – First and Only PAO Compliant eFRM Media HEPA Filter
The purpose of installed HEPA filter integrity testing, also called in-situ testing, is to confirm a flawless performance during normal operation. With AAF Flanders’ new eFRM Media Technology, MEGAcel II filters can be scan tested with the industry standard photometer at the standard aerosol concentrations, as well as the low-aerosol concentration Discrete Particle Counter (DPC) method.
The MEGAcel II filter contains dual-density eFRM Media Technology media specifically developed to retain equivalent amounts of PAO aerosol with equivalent or lower pressure drop increases as ultrafine microglass. The dual-density eFRM layers allow for the in-depth capture of progressively smaller solid particles.
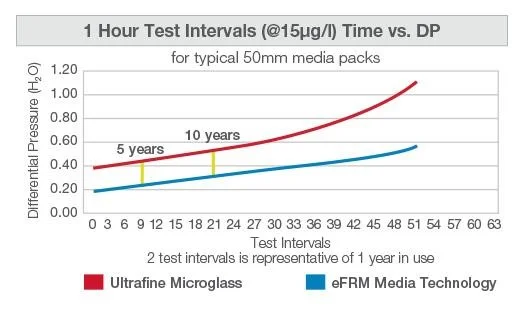
Independent laboratory studies have shown that MEGAcel II eFRM Media Technology have compatible PAO holding capacity as compared to traditional ultrafine microglass HEPA media, while providing significant reduction in system resistance, which equates to energy savings, as seen in the results below.
Enhanced Chemical Tolerance
High Corrosion Resistance
eFRM Media Technology is proven to be resistant in highly corrosive environments, and will withstand attacks from common decontamination chemicals. Both components of the eFRM Media Technology, the membrane and non-woven layers, are stable against exposure at the prescribed time and concentration for the above disinfectant agents.
Superior Water Resistance
Based on AAF Flanders’ test lab results, eFRM Media Technology provides superior water resistance in comparison with ultrafine microglass, reducing damage risk.
Negligible Off-Gassing
eFRM Media Technology has extremely low off-gassing of chemical components, resulting in the highest quality clean air available.